Composition and reactivity of organic aerosols
About 30-50% of atmospheric aerosol particles are composed of organic material and often the majority of these organic aerosol particles are formed within the atmosphere from gaseous organic precursors. Despite their dominance, the chemical composition, formation pathways, and atmospheric reactions and effects of these organic particles are poorly characterised, mainly due to their highly complex composition with more than 10 000 organic compounds present in organic aerosol covering a wide physical-chemical parameter space. The analysis of these highly complex mixtures at trace level concentrations is a main challenge in the study of atmospheric aerosols. To better characterise aerosol effects on climate and human health an improved understanding of aerosol composition, evolution and sources is needed, which is only possible through an improved knowledge of particle composition.
We are using a range of laboratory experimental techniques to study fundamental aspects of aerosols formation and composition and we are characterising aerosol and rain samples collected in the ambient atmosphere ranging from polluted urban to clean remote locations.
(1) Detailed, molecular-level analysis of organic aerosol composition. To gain detailed insights into the chemical composition of organic aerosols, we are using a number of state-of-the-art analytical-chemical techniques, including ultra-high resolution mass spectrometry, chromatographic and optical spectroscopy methods. We are also developing new techniques to obtain a comprehensive picture of the composition and evolution of organic aerosols.
We are mainly using ultra-high resolution mass spectrometry (UHR-MS) coupled to different ionisation techniques to characterise aerosol composition. This technique is capable of assigning the elemental composition of 1000s of unknown peaks detected in mass spectra of complex mixtures such as organic aerosols allowing to identify overall compositional differences between samples and to link to atmospheric processes and sources such as cloud formation or biological activities.
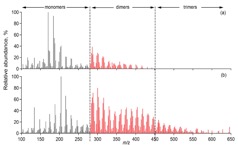
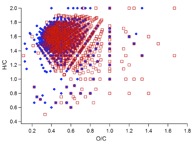
Figure 1. (a) Two ultra-high resolution mass spectra (UHR-MS) of organic aerosol particles collected at a boreal forest site in Finland (Hyytiälä). The particle composition, especially its high molecular weight fraction, can vary significantly depending on environmental factors such as temperature, oxidation regime or biogenic emissions. (b) UHR-MS of organic aerosol generated in laboratory experiments. Each data point represents a peak in the mass spectrum with their atomic ratio of H/C plotted vs. O/C. Blue diamonds represent the composition of aerosol formed and oxidised with ozone and red squares shows the composition of particles formed in the presence of OH, leading to a clearly more highly oxidised average particle composition with potential consequences on particle-cloud interactions and other atmospheric effects.
(2) Aerosol Extractive Electrospray Ionisation Mass Spectrometry (MS). We developed an online MS ionisation source (Extractive Electrospray Ionisation, EESI) which allows to quantify organic and inorganic compounds with minimal fragmentation and matrix effects, from which other soft ionisation techniques often suffer. Particle water content and inorganic components such as ammonium sulphate do not affect EESI quantification results making it an ideal tool to quantify aerosol composition changes with high time resolution.
This new MS ionisation technique allows to characterise particle composition at a molecular level and follow changes in particle composition with a time resolution of a few seconds to minutes. We are using EESI-MS for laboratory experiments to study aerosol composition changes when exposed to atmospheric oxidants and to characterise kinetic and mechanisms of particle phase reaction.
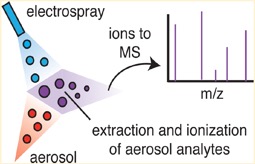
Figure 2. Schematic showing the EESI ionisation technique. A pure solvent electrospray (blue) collides with an aerosol flow (red) leading to a quantitative extraction and ionisation of aerosol components. This soft ionisation technique leads to minimal fragmentation and thus is ideal to characterise complex organic mixtures.
(3) Criegee Intermediates. A significant fraction of organic particles is composed of low-volatility oxidation products of gaseous compounds emitted by vegetation such as terpenes or isoprene. These alkenes are efficiently oxidised by ozone initiating a complex oxidation cascade. The first reactive intermediates of the reaction between ozone and alkenes are so-called Criegee Intermediates (CIs) with bi-radical structure. A detailed understanding of the fate and reactivity of CIs in the atmosphere is essential for many aspects of atmospheric chemistry but characterisation and quantification of CIs is highly challenging due to their short lifetime. We developed a novel and universal method to stabilise and quantify CIs under atmospheric conditions based on online Proton Transfer Reaction Mass Spectrometry (PTR-MS) where the short-lived CIs are selectively reacted and stabilised with spin traps to allow online and offline chemical characterisation.
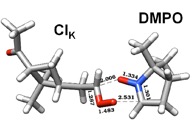
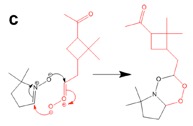
Figure 3. DFT calculation and proposed mechanism of the reaction of the spin trap DMPO with a Criegee Intermediate of a-pinene forming a stable six-membered ring.
(4) Particle reactivity and particle phase. Atmospheric trace gases react with aerosol particle surfaces or bulk with effects on gas and particle composition and phase. We are studying heterogeneous reaction kinetics of trace gases such as ozone, nitrogen oxides on mineral particles as surrogates of desert dust particles assessing the changes of these trace gases due to heterogeneous aerosol reactions.
Heterogeneous reactions of oxidants such as ozone or OH radicals can alos change the composition of organic particle, which affects their phase leading to particles with higher viscosity. Higher viscosity particles may lead to slower water uptake and altered aerosol-cloud interactions.
In collaboration with Dr Francis Pope (University of Birmingham) and Dr Marina Kuimova (Imperial College London) we developed a new technique to quantify particle viscosity using molecular rotors and fluorescence lifetime imaging (FLIM), which allows tracking viscosity at a sub-micron scale and with seconds time resolution.
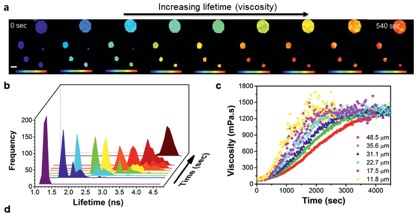
Figure 4. (a) Time-dependent FLIM imaging of oleic acid droplets upon exposure to ozone. Changes in particle composition due to oleic acid oxidation lead to an increase in fluorescence lifetime (b) and particle viscosity (c).
Related Publications
- Kourtchev I. et al., Enhanced Volatile Organic Compounds emissions and organic aerosol mass increase the oligomer content of atmospheric aerosols, Nature Scientific Reports, 6, DOI: 10.1038/srep35038, 2016.
- Kourtchev I. et al., Molecular composition of organic aerosols in central Amazonia: an ultra-high resolution mass spectrometry study, Atmos. Chem. Phys, 16, 11899–11913, 2016.
- Gallimore P.J. and M. Kalberer, Characterising an Extractive Electrospray Ionisation (EESI) source for the online mass spectrometry analysis of organic aerosols, Environ. Sci. Technol. 47, 7324−7331, 2013.
- Gallimore, P.J. et al., Comprehensive modeling study of ozonolysis of oleic acid aerosol based on real-time, online measurements of aerosol composition, J. Geophys. Res. Atmos., 122, doi:10.1002/2016JD026221, 2017.
- Giorio C. et al., Online Quantification of Criegee Intermediates of α‑Pinene Ozonolysis by Stabilization with Spin Traps and Proton-Transfer Reaction Mass Spectrometry Detection, J. Am. Chem. Soc., 139, 3999−4008,2017.
- Tang M. et al., Heterogeneous reaction of ClONO2 with TiO2 and SiO2 aerosol particles: implications for stratospheric particle injection for climate engineering, Atmos. Chem. Phys., 16, 15397–15412, 2016.
- Tang M. et al., Heterogeneous Interaction of SiO2 with N2O5: Aerosol Flow Tube and Single Particle Optical Levitation−Raman Spectroscopy Studies, J. Phys. Chem. A, 118, 8817−8827, 2014.
- Fitzgerald C. et al., Fluorescence lifetime imaging of optically levitated aerosol: a technique to quantitatively map the viscosity of suspended aerosol particles. Phys. Chem. Chem. Phys., 18, 21710, 2016.
- Hosny N.A. et al., Direct imaging of changes in aerosol particle viscosity upon hydration and chemical aging, Chem. Sci., 7, 1357–1367, 2016.
Quick Links
Social Media
