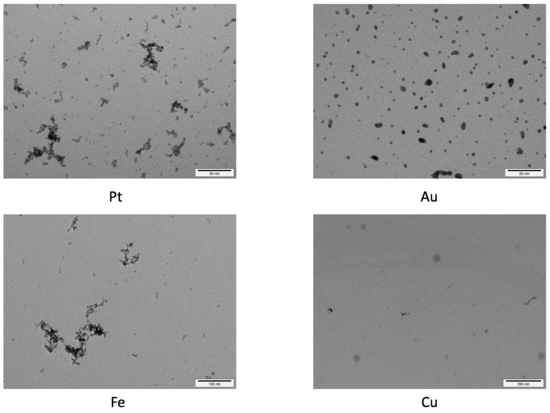
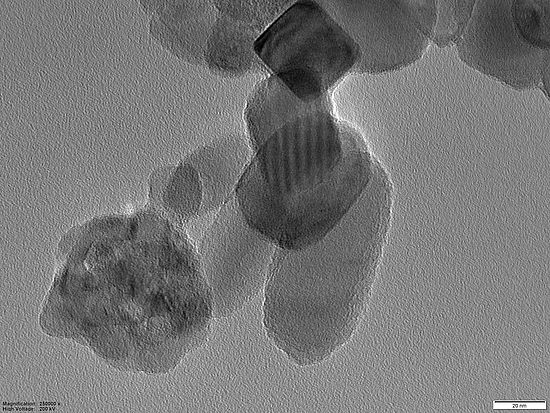
Metal Nanoparticles
In our group, we work on the generation of metal nanoparticles via spark ablation. This method ensures a generation of particles within the size range of 1-10 nm and allow coagulation of the metal nanoparticle with other particles such as titanium dioxide. Characterization of nanoparticles of this size is challenging and, therefore, in our group we develop new quantification analysis approaches.
Our research includes the synthesis of nanoparticles, their characterization and application. Our characterization techniques include e.g., electron microscopy, X-ray powder diffraction and mass-spectrometry.
Quick Links
Social Media
